Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats
Carlos Chaccour, Gloria Abizanda, Ángel Irigoyen-Barrio, Aina Casellas, Azucena Aldaz, Fernando Martínez-Galán, Felix Hammann, Ana Gloria Gil
Scientific Reports, doi:10.1038/s41598-020-74084-y
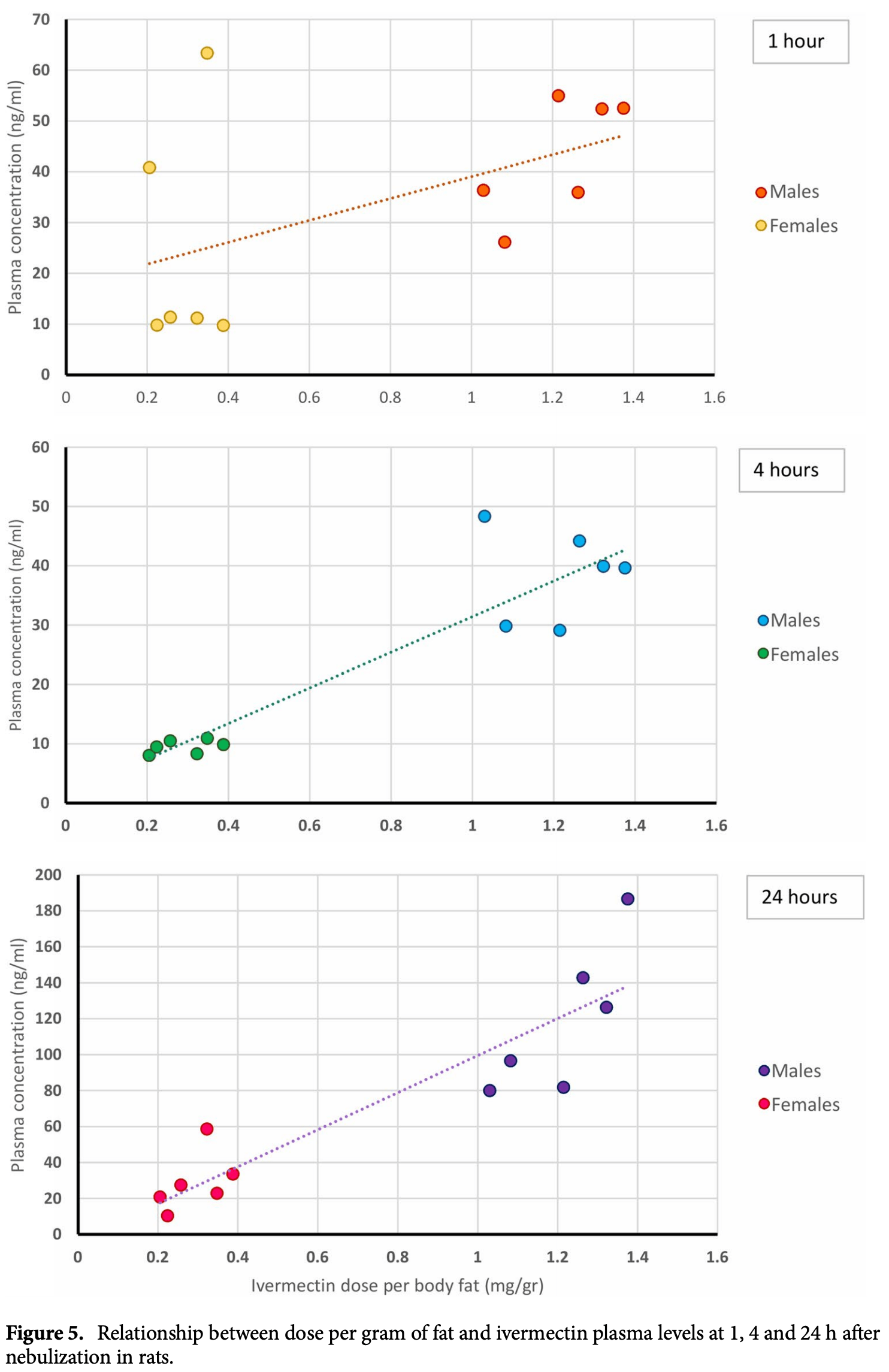
Ivermectin is a widely used antiparasitic drug with known efficacy against several single-strain RNA viruses. Recent data shows significant reduction of SARS-CoV-2 replication in vitro by ivermectin concentrations not achievable with safe doses orally. Inhaled therapy has been used with success for other antiparasitics. An ethanol-based ivermectin formulation was administered once to 14 rats using a nebulizer capable of delivering particles with alveolar deposition. Rats were randomly assigned into three target dosing groups, lower dose (80-90 mg/kg), higher dose (110-140 mg/kg) or ethanol vehicle only. A toxicology profile including behavioral and weight monitoring, full blood count, biochemistry, necropsy and histological examination of the lungs was conducted. The pharmacokinetic profile of ivermectin in plasma and lungs was determined in all animals. There were no relevant changes in behavior or body weight. There was a delayed elevation in muscle enzymes compatible with rhabdomyolysis, that was also seen in the control group and has been attributed to the ethanol dose which was up to 11 g/kg in some animals. There were no histological anomalies in the lungs of any rat. Male animals received a higher ivermectin dose adjusted by adipose weight and reached higher plasma concentrations than females in the same dosing group (mean C max 86.2 ng/ml vs. 26.2 ng/ ml in the lower dose group and 152 ng/ml vs. 51.8 ng/ml in the higher dose group). All subjects had detectable ivermectin concentrations in the lungs at seven days post intervention, up to 524.3 ng/g for high-dose male and 27.3 ng/g for low-dose females. nebulized ivermectin can reach pharmacodynamic concentrations in the lung tissue of rats, additional experiments are required to assess the safety of this formulation in larger animals. As of August 19, 2020, there have been more than 22 million COVID-19 cases causing over 785,000 deaths worldwide. In the absence of a vaccine, numerous efforts are ongoing to develop drug-based strategies to prevent, treat or reduce the transmission of the virus. Data on several drug regimens suggest lack of efficacy for lopinavirritonavir 1 , hydroxychloroquine as prophylaxis 2 or even harmfulness such as high-dose hydroxychloroquine for prophylaxis 3 while remdesivir 4 and dexamethasone 5 seem to improve patients' outcome. Ivermectin is a widely used antiparasitic drug with known efficacy against several single-strain RNA viruses including Dengue 6 , Zika 7 and other viruses 8 . The effect on flaviviruses could be explained by a reduction of the viral penetration into the nucleus via an effect on the host´s importin alpha/beta1 9 , inhibition of the viral helicase 8 or yet to be described mechanisms. Caly et al. showed a significant reduction of SARS-CoV-2 replication after incubating Vero cells, a cell line derived from African Green Monkey kidney epithelial cells, for 48 h with ivermectin concentrations not readily attainable in vivo 10 .
Author contributions
Competing interests The authors declare no competing interests.
References
Baraka, Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus, Eur. J. Clin. Pharmacol
Borba, Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial, JAMA Netw. Open,
doi:10.1001/jamanetworkopen.2020.8857Boulware, A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19, N. Engl. J. Med,
doi:10.1056/NEJMoa2016638Chaccour, Hammann, Ramon-Garcia, Rabinovich, Ivermectin and novel coronavirus disease (COVID-19): keeping rigor in times of urgency, Am. J. Trop. Med. Hyg,
doi:10.4269/ajtmh.20-0271Changeux, Amoura, Rey, Miyara, A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications, C. R. Biol,
doi:10.5802/crbiol.8Ci, Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogenactivated protein kinase activation pathway, Fundam. Clin. Pharmacol,
doi:10.1111/j.1472-8206.2009.00684.xEl-Khatib, Lehnert, Lung density changes observed in vivo in rat lungs after irradiation: variations among and within individual lungs, Int. J. Radiat. Oncol. Biol. Phys,
doi:10.1016/0360-3016(89)90494-xEraslan, Comparative pharmacokinetics of some injectable preparations containing ivermectin in dogs, Food Chem. Toxicol,
doi:10.1016/j.fct.2010.05.043Ferrell, Koong, Influence of plane of nutrition on body composition, organ size and energy utilization of Sprague-Dawley rats, J. Nutr,
doi:10.1093/jn/116.12.2525Gil, Silvan, Illera, Illera, The effects of anesthesia on the clinical chemistry of New Zealand White rabbits, Contemp. Top. Lab. Anim. Sci
Krause, Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor, Mol. Pharmacol,
doi:10.1124/mol.53.2.283Lei, Study on the subacute inhalation toxicity of Ivermectin TC in rats, Chin. J. Comp. Med
Maclean, Valentine, Jatlow, Sofuoglu, Inhalation of alcohol vapor: measurement and implications, Alcohol Clin. Exp. Res,
doi:10.1111/acer.13291Mastrangelo, Ivermectin a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J. Antimicrob. Chemother,
doi:10.1093/jac/dks147Mathiasen, Moser, The Irwin test and functional observational battery (FOB) for assessing the effects of compounds on behavior, physiology, and safety pharmacology in rodents, Curr. Protoc. Pharmacol,
doi:10.1002/cpph.43Mcivor, Berger, Pack, Rachlis, Chan, An effectiveness community-based clinical trial of Respirgard II and Fisoneb nebulizers for Pneumocystis carinii prophylaxis with aerosol pentamidine in HIV-infected individuals. Toronto Aerosol Pentamidine Study (TAPS) Group, Chest,
doi:10.1378/chest.110.1.141Melotti, The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer, EMBO Mol. Med,
doi:10.15252/emmm.201404084Montgomery, Aerosolised pentamidine as sole therapy for Pneumocystis carinii pneumonia in patients with acquired immunodeficiency syndrome, Lancet,
doi:10.1016/s0140-6736(87)91794-6Nishiyama, Yokoyama, Hanaoka, Liver function after sevoflurane or isoflurane anaesthesia in neurosurgical patients, Can. J. Anaesth,
doi:10.1007/BF03012146Ouedraogo, Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial, Clin. Infect. Dis,
doi:10.1093/cid/ciu797Pacanowski, Subcutaneous ivermectin as a safe salvage therapy in Strongyloides stercoralis hyperinfection syndrome: a case report, Am. J. Trop. Med. Hyg
Papadatos, Nontraumatic rhabdomyolysis with short-term alcohol intoxication-a case report, Clin. Case Rep,
doi:10.1002/ccr3.326Salluh, Successful use of parenteral ivermectin in an immunosuppressed patient with disseminated strongyloidiasis and septic shock, Intensive Care Med
Sutherland, Leathwick, Anthelmintic resistance in nematode parasites of cattle: a global issue?, Trends Parasitol,
doi:10.1016/j.pt.2010.11.008Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin alpha/betamediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem. J,
doi:10.1042/BJ20120150Wiberg, Trenholm, Coldwell, Increased ethanol toxicity in old rats: changes in LD50, in vivo and in vitro metabolism, and liver alcohol dehydrogenase activity, Toxicol. Appl. Pharmacol,
doi:10.1016/0041-008x(70)90077-3Yang, Tekwani, Martin, In COVID-19, adding lopinavir-ritonavir to usual care did not shorten time to clinical improvement, Ann. Intern. Med,
doi:10.7326/ACPJ202006160-063Zhang, Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice, Inflamm. Res,
doi:10.1007/s00011-008-8007-8{ 'indexed': {'date-parts': [[2024, 4, 15]], 'date-time': '2024-04-15T23:13:53Z', 'timestamp': 1713222833183},
'reference-count': 41,
'publisher': 'Springer Science and Business Media LLC',
'issue': '1',
'license': [ { 'start': { 'date-parts': [[2020, 10, 13]],
'date-time': '2020-10-13T00:00:00Z',
'timestamp': 1602547200000},
'content-version': 'tdm',
'delay-in-days': 0,
'URL': 'https://creativecommons.org/licenses/by/4.0'},
{ 'start': { 'date-parts': [[2020, 10, 13]],
'date-time': '2020-10-13T00:00:00Z',
'timestamp': 1602547200000},
'content-version': 'vor',
'delay-in-days': 0,
'URL': 'https://creativecommons.org/licenses/by/4.0'}],
'funder': [ { 'DOI': '10.13039/501100004435',
'name': 'Universidad de Navarra',
'doi-asserted-by': 'publisher'}],
'content-domain': {'domain': ['link.springer.com'], 'crossmark-restriction': False},
'abstract': '<jats:title>Abstract</jats:title><jats:p>Ivermectin is a widely used antiparasitic drug with '
'known efficacy against several single-strain RNA viruses. Recent data shows significant '
'reduction of SARS-CoV-2 replication in vitro by ivermectin concentrations not achievable with '
'safe doses orally. Inhaled therapy has been used with success for other antiparasitics. An '
'ethanol-based ivermectin formulation was administered once to 14 rats using a nebulizer '
'capable of delivering particles with alveolar deposition. Rats were randomly assigned into '
'three target dosing groups, lower dose (80–90\xa0mg/kg), higher dose (110–140\xa0mg/kg) or '
'ethanol vehicle only. A toxicology profile including behavioral and weight monitoring, full '
'blood count, biochemistry, necropsy and histological examination of the lungs was conducted. '
'The pharmacokinetic profile of ivermectin in plasma and lungs was determined in all animals. '
'There were no relevant changes in behavior or body weight. There was a delayed elevation in '
'muscle enzymes compatible with rhabdomyolysis, that was also seen in the control group and '
'has been attributed to the ethanol dose which was up to 11\xa0g/kg in some animals. There '
'were no histological anomalies in the lungs of any rat. Male animals received a higher '
'ivermectin dose adjusted by adipose weight and reached higher plasma concentrations than '
'females in the same dosing group (mean C<jats:sub>max</jats:sub> 86.2\xa0ng/ml vs. 26.2\xa0'
'ng/ml in the lower dose group and 152\xa0ng/ml vs. 51.8\xa0ng/ml in the higher dose group). '
'All subjects had detectable ivermectin concentrations in the lungs at seven days post '
'intervention, up to 524.3\xa0ng/g for high-dose male and 27.3\xa0ng/g for low-dose females. '
'nebulized ivermectin can reach pharmacodynamic concentrations in the lung tissue of rats, '
'additional experiments are required to assess the safety of this formulation in larger '
'animals.</jats:p>',
'DOI': '10.1038/s41598-020-74084-y',
'type': 'journal-article',
'created': { 'date-parts': [[2020, 10, 13]],
'date-time': '2020-10-13T10:05:00Z',
'timestamp': 1602583500000},
'update-policy': 'http://dx.doi.org/10.1007/springer_crossmark_policy',
'source': 'Crossref',
'is-referenced-by-count': 29,
'title': 'Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, '
'dose-ranging study in rats',
'prefix': '10.1038',
'volume': '10',
'author': [ {'given': 'Carlos', 'family': 'Chaccour', 'sequence': 'first', 'affiliation': []},
{'given': 'Gloria', 'family': 'Abizanda', 'sequence': 'additional', 'affiliation': []},
{'given': 'Ángel', 'family': 'Irigoyen-Barrio', 'sequence': 'additional', 'affiliation': []},
{'given': 'Aina', 'family': 'Casellas', 'sequence': 'additional', 'affiliation': []},
{'given': 'Azucena', 'family': 'Aldaz', 'sequence': 'additional', 'affiliation': []},
{'given': 'Fernando', 'family': 'Martínez-Galán', 'sequence': 'additional', 'affiliation': []},
{'given': 'Felix', 'family': 'Hammann', 'sequence': 'additional', 'affiliation': []},
{'given': 'Ana Gloria', 'family': 'Gil', 'sequence': 'additional', 'affiliation': []}],
'member': '297',
'published-online': {'date-parts': [[2020, 10, 13]]},
'reference': [ { 'key': '74084_CR1',
'doi-asserted-by': 'publisher',
'first-page': 'JC63',
'DOI': '10.7326/ACPJ202006160-063',
'volume': '172',
'author': 'P Yang',
'year': '2020',
'unstructured': 'Yang, P., Tekwani, S. & Martin, G. S. In COVID-19, adding '
'lopinavir-ritonavir to usual care did not shorten time to clinical '
'improvement. Ann. Intern. Med. 172, JC63. '
'https://doi.org/10.7326/ACPJ202006160-063 (2020).',
'journal-title': 'Ann. Intern. Med.'},
{ 'key': '74084_CR2',
'doi-asserted-by': 'publisher',
'DOI': '10.1056/NEJMoa2016638',
'author': 'DR Boulware',
'year': '2020',
'unstructured': 'Boulware, D. R. et al. A randomized trial of hydroxychloroquine as '
'postexposure prophylaxis for covid-19. N. Engl. J. Med. '
'https://doi.org/10.1056/NEJMoa2016638 (2020).',
'journal-title': 'N. Engl. J. Med.'},
{ 'key': '74084_CR3',
'doi-asserted-by': 'publisher',
'first-page': 'e208857',
'DOI': '10.1001/jamanetworkopen.2020.8857',
'volume': '3',
'author': 'MGS Borba',
'year': '2020',
'unstructured': 'Borba, M. G. S. et al. Effect of high vs low doses of chloroquine '
'diphosphate as adjunctive therapy for patients hospitalized with severe '
'acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a '
'randomized clinical trial. JAMA Netw. Open 3, e208857. '
'https://doi.org/10.1001/jamanetworkopen.2020.8857 (2020).',
'journal-title': 'JAMA Netw. Open'},
{ 'key': '74084_CR4',
'doi-asserted-by': 'publisher',
'DOI': '10.1056/NEJMoa2007764',
'author': 'JH Beigel',
'year': '2020',
'unstructured': 'Beigel, J. H. et al. Remdesivir for the treatment of '
'covid-19—preliminary report. N. Engl. J. Med. '
'https://doi.org/10.1056/NEJMoa2007764 (2020).',
'journal-title': 'N. Engl. J. Med.'},
{ 'key': '74084_CR5',
'doi-asserted-by': 'publisher',
'DOI': '10.1101/2020.06.22.20137273',
'author': 'P Horby',
'year': '2020',
'unstructured': 'Horby, P. et al. Effect of dexamethasone in hospitalized patients with '
'COVID-19: preliminary report. medRxiv '
'https://doi.org/10.1101/2020.06.22.20137273 (2020).',
'journal-title': 'medRxiv'},
{ 'key': '74084_CR6',
'doi-asserted-by': 'publisher',
'first-page': '851',
'DOI': '10.1042/BJ20120150',
'volume': '443',
'author': 'KM Wagstaff',
'year': '2012',
'unstructured': 'Wagstaff, K. M., Sivakumaran, H., Heaton, S. M., Harrich, D. & Jans, D. '
'A. Ivermectin is a specific inhibitor of importin alpha/beta-mediated '
'nuclear import able to inhibit replication of HIV-1 and dengue virus. '
'Biochem. J. 443, 851–856. https://doi.org/10.1042/BJ20120150 (2012).',
'journal-title': 'Biochem. J.'},
{ 'key': '74084_CR7',
'doi-asserted-by': 'publisher',
'first-page': '259',
'DOI': '10.1016/j.chom.2016.07.004',
'volume': '20',
'author': 'NJ Barrows',
'year': '2016',
'unstructured': 'Barrows, N. J. et al. A screen of FDA-approved drugs for inhibitors of '
'Zika virus infection. Cell Host Microbe 20, 259–270. '
'https://doi.org/10.1016/j.chom.2016.07.004 (2016).',
'journal-title': 'Cell Host Microbe'},
{ 'key': '74084_CR8',
'doi-asserted-by': 'publisher',
'first-page': '1884',
'DOI': '10.1093/jac/dks147',
'volume': '67',
'author': 'E Mastrangelo',
'year': '2012',
'unstructured': 'Mastrangelo, E. et al. Ivermectin is a potent inhibitor of flavivirus '
'replication specifically targeting NS3 helicase activity: new prospects '
'for an old drug. J. Antimicrob. Chemother. 67, 1884–1894. '
'https://doi.org/10.1093/jac/dks147 (2012).',
'journal-title': 'J. Antimicrob. Chemother.'},
{ 'key': '74084_CR9',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.antiviral.2020.104760',
'author': 'SNY Yang',
'year': '2020',
'unstructured': 'Yang, S. N. Y. et al. The broad spectrum antiviral ivermectin targets '
'the host nuclear transport importin alpha/beta1 heterodimer. Antiviral '
'Res. https://doi.org/10.1016/j.antiviral.2020.104760 (2020).',
'journal-title': 'Antiviral Res.'},
{ 'key': '74084_CR10',
'doi-asserted-by': 'publisher',
'DOI': '10.1016/j.antiviral.2020.104787',
'author': 'L Caly',
'year': '2020',
'unstructured': 'Caly, L., Druce, J., Catton, M., Jans, D. & Wagstaff, K. M. The '
'FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in '
'vitro. Antivir. Res. https://doi.org/10.1016/j.antiviral.2020.104787 '
'(2020) (in Press, Journal Pre-proof).',
'journal-title': 'Antivir. Res.'},
{ 'key': '74084_CR11',
'doi-asserted-by': 'publisher',
'DOI': '10.4269/ajtmh.20-0271',
'author': 'C Chaccour',
'year': '2020',
'unstructured': 'Chaccour, C., Hammann, F., Ramon-Garcia, S. & Rabinovich, N. R. '
'Ivermectin and novel coronavirus disease (COVID-19): keeping rigor in '
'times of urgency. Am. J. Trop. Med. Hyg. '
'https://doi.org/10.4269/ajtmh.20-0271 (2020).',
'journal-title': 'Am. J. Trop. Med. Hyg.'},
{ 'key': '74084_CR12',
'doi-asserted-by': 'publisher',
'first-page': '524',
'DOI': '10.1007/s00011-008-8007-8',
'volume': '57',
'author': 'X Zhang',
'year': '2008',
'unstructured': 'Zhang, X. et al. Ivermectin inhibits LPS-induced production of '
'inflammatory cytokines and improves LPS-induced survival in mice. '
'Inflamm. Res. 57, 524–529. https://doi.org/10.1007/s00011-008-8007-8 '
'(2008).',
'journal-title': 'Inflamm. Res.'},
{ 'key': '74084_CR13',
'doi-asserted-by': 'publisher',
'first-page': '449',
'DOI': '10.1111/j.1472-8206.2009.00684.x',
'volume': '23',
'author': 'X Ci',
'year': '2009',
'unstructured': 'Ci, X. et al. Avermectin exerts anti-inflammatory effect by '
'downregulating the nuclear transcription factor kappa-B and '
'mitogen-activated protein kinase activation pathway. Fundam. Clin. '
'Pharmacol. 23, 449–455. https://doi.org/10.1111/j.1472-8206.2009.00684.x '
'(2009).',
'journal-title': 'Fundam. Clin. Pharmacol.'},
{ 'key': '74084_CR14',
'doi-asserted-by': 'publisher',
'DOI': '10.5802/crbiol.8',
'author': 'J Changeux',
'year': '2020',
'unstructured': 'Changeux, J., Amoura, Z., Rey, F. & Miyara, M. A nicotinic hypothesis '
'for Covid-19 with preventive and therapeutic implications. C. R. Biol. '
'https://doi.org/10.5802/crbiol.8 (2020).',
'journal-title': 'C. R. Biol.'},
{ 'key': '74084_CR15',
'doi-asserted-by': 'publisher',
'first-page': '2001116',
'DOI': '10.1183/13993003.01116-2020',
'volume': '55',
'author': 'P Russo',
'year': '2020',
'unstructured': 'Russo, P. et al. COVID-19 and smoking: is nicotine the hidden link?. '
'Eur. Respir. J. 55, 2001116. https://doi.org/10.1183/13993003.01116-2020 '
'(2020).',
'journal-title': 'Eur. Respir. J.'},
{ 'key': '74084_CR16',
'doi-asserted-by': 'publisher',
'first-page': '283',
'DOI': '10.1124/mol.53.2.283',
'volume': '53',
'author': 'RM Krause',
'year': '1998',
'unstructured': 'Krause, R. M. et al. Ivermectin: a positive allosteric effector of the '
'alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 53, '
'283–294. https://doi.org/10.1124/mol.53.2.283 (1998).',
'journal-title': 'Mol. Pharmacol.'},
{ 'key': '74084_CR17',
'doi-asserted-by': 'publisher',
'first-page': '480',
'DOI': '10.1016/s0140-6736(87)91794-6',
'volume': '2',
'author': 'AB Montgomery',
'year': '1987',
'unstructured': 'Montgomery, A. B. et al. Aerosolised pentamidine as sole therapy for '
'Pneumocystis carinii pneumonia in patients with acquired '
'immunodeficiency syndrome. Lancet 2, 480–483. '
'https://doi.org/10.1016/s0140-6736(87)91794-6 (1987).',
'journal-title': 'Lancet'},
{ 'key': '74084_CR18',
'doi-asserted-by': 'publisher',
'first-page': '141',
'DOI': '10.1378/chest.110.1.141',
'volume': '110',
'author': 'RA McIvor',
'year': '1996',
'unstructured': 'McIvor, R. A., Berger, P., Pack, L. L., Rachlis, A. & Chan, C. K. An '
'effectiveness community-based clinical trial of Respirgard II and '
'Fisoneb nebulizers for Pneumocystis carinii prophylaxis with aerosol '
'pentamidine in HIV-infected individuals. Toronto Aerosol Pentamidine '
'Study (TAPS) Group. Chest 110, 141–146. '
'https://doi.org/10.1378/chest.110.1.141 (1996).',
'journal-title': 'Chest'},
{ 'key': '74084_CR19',
'first-page': '70',
'volume': '26',
'author': 'JI Lei',
'year': '2016',
'unstructured': 'Lei, J. I. et al. Study on the subacute inhalation toxicity of '
'Ivermectin TC in rats. Chin. J. Comp. Med. 26, 70–74 (2016).',
'journal-title': 'Chin. J. Comp. Med.'},
{ 'key': '74084_CR20',
'doi-asserted-by': 'publisher',
'first-page': '97',
'DOI': '10.1016/j.actatropica.2013.03.019',
'volume': '127',
'author': 'MM Homeida',
'year': '2013',
'unstructured': 'Homeida, M. M. et al. The lack of influence of food and local alcoholic '
'brew on the blood level of Mectizan((R)) (ivermectin). Acta Trop. 127, '
'97–100. https://doi.org/10.1016/j.actatropica.2013.03.019 (2013).',
'journal-title': 'Acta Trop.'},
{ 'key': '74084_CR21',
'unstructured': 'FDA. Center for drug evaluation and research. Approval package for '
'Mectizan. '
'https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/050742ap.pdf. '
'Accessed July 2016.'},
{ 'key': '74084_CR22',
'doi-asserted-by': 'publisher',
'first-page': 'e43',
'DOI': '10.1002/cpph.43',
'volume': '83',
'author': 'JR Mathiasen',
'year': '2018',
'unstructured': 'Mathiasen, J. R. & Moser, V. C. The Irwin test and functional '
'observational battery (FOB) for assessing the effects of compounds on '
'behavior, physiology, and safety pharmacology in rodents. Curr. Protoc. '
'Pharmacol. 83, e43. https://doi.org/10.1002/cpph.43 (2018).',
'journal-title': 'Curr. Protoc. Pharmacol.'},
{ 'key': '74084_CR23',
'doi-asserted-by': 'publisher',
'first-page': '2181',
'DOI': '10.1016/j.fct.2010.05.043',
'volume': '48',
'author': 'G Eraslan',
'year': '2010',
'unstructured': 'Eraslan, G. et al. Comparative pharmacokinetics of some injectable '
'preparations containing ivermectin in dogs. Food Chem. Toxicol. 48, '
'2181–2185. https://doi.org/10.1016/j.fct.2010.05.043 (2010).',
'journal-title': 'Food Chem. Toxicol.'},
{ 'key': '74084_CR24',
'doi-asserted-by': 'publisher',
'first-page': '327',
'DOI': '10.1016/s0304-4017(99)00175-2',
'volume': '87',
'author': 'A Lifschitz',
'year': '2000',
'unstructured': 'Lifschitz, A. et al. Comparative distribution of ivermectin and '
'doramectin to parasite location tissues in cattle. Vet. Parasitol. 87, '
'327–338. https://doi.org/10.1016/s0304-4017(99)00175-2 (2000).',
'journal-title': 'Vet. Parasitol.'},
{ 'key': '74084_CR25',
'doi-asserted-by': 'publisher',
'first-page': '407',
'DOI': '10.1007/s002280050131',
'volume': '50',
'author': 'OZ Baraka',
'year': '1996',
'unstructured': 'Baraka, O. Z. et al. Ivermectin distribution in the plasma and tissues '
'of patients infected with Onchocerca volvulus. Eur. J. Clin. Pharmacol. '
'50, 407–410 (1996).',
'journal-title': 'Eur. J. Clin. Pharmacol.'},
{ 'key': '74084_CR26',
'doi-asserted-by': 'publisher',
'first-page': '357',
'DOI': '10.1093/cid/ciu797',
'volume': '60',
'author': 'AL Ouedraogo',
'year': '2015',
'unstructured': 'Ouedraogo, A. L. et al. Efficacy and safety of the mosquitocidal drug '
'ivermectin to prevent malaria transmission after treatment: a '
'double-blind, randomized, clinical trial. Clin. Infect. Dis. 60, '
'357–365. https://doi.org/10.1093/cid/ciu797 (2015).',
'journal-title': 'Clin. Infect. Dis.'},
{ 'key': '74084_CR27',
'doi-asserted-by': 'publisher',
'first-page': '2525',
'DOI': '10.1093/jn/116.12.2525',
'volume': '116',
'author': 'CL Ferrell',
'year': '1986',
'unstructured': 'Ferrell, C. L. & Koong, K. J. Influence of plane of nutrition on body '
'composition, organ size and energy utilization of Sprague-Dawley rats. '
'J. Nutr. 116, 2525–2535. https://doi.org/10.1093/jn/116.12.2525 (1986).',
'journal-title': 'J. Nutr.'},
{ 'key': '74084_CR28',
'doi-asserted-by': 'publisher',
'first-page': '745',
'DOI': '10.1016/0360-3016(89)90494-x',
'volume': '16',
'author': 'E El-Khatib',
'year': '1989',
'unstructured': 'El-Khatib, E. & Lehnert, S. Lung density changes observed in vivo in rat '
'lungs after irradiation: variations among and within individual lungs. '
'Int. J. Radiat. Oncol. Biol. Phys. 16, 745–754. '
'https://doi.org/10.1016/0360-3016(89)90494-x (1989).',
'journal-title': 'Int. J. Radiat. Oncol. Biol. Phys.'},
{ 'key': '74084_CR29',
'doi-asserted-by': 'publisher',
'first-page': '718',
'DOI': '10.1016/0041-008x(70)90077-3',
'volume': '16',
'author': 'GS Wiberg',
'year': '1970',
'unstructured': 'Wiberg, G. S., Trenholm, H. L. & Coldwell, B. B. Increased ethanol '
'toxicity in old rats: changes in LD50, in vivo and in vitro metabolism, '
'and liver alcohol dehydrogenase activity. Toxicol. Appl. Pharmacol. 16, '
'718–727. https://doi.org/10.1016/0041-008x(70)90077-3 (1970).',
'journal-title': 'Toxicol. Appl. Pharmacol.'},
{ 'key': '74084_CR30',
'doi-asserted-by': 'publisher',
'first-page': 'P94',
'DOI': '10.1016/S0009-9236(03)90702-8',
'volume': '73',
'author': 'SR Vanapalli',
'year': '2003',
'unstructured': 'Vanapalli, S. R. et al. Orange juice decreases the oral bioavailability '
'of ivermectin in healthy volunteers. Clin. Pharmacol. Ther. 73, P94–P94. '
'https://doi.org/10.1016/S0009-9236(03)90702-8 (2003).',
'journal-title': 'Clin. Pharmacol. Ther.'},
{ 'key': '74084_CR31',
'doi-asserted-by': 'publisher',
'first-page': '769',
'DOI': '10.1002/ccr3.326',
'volume': '3',
'author': 'SS Papadatos',
'year': '2015',
'unstructured': 'Papadatos, S. S. et al. Nontraumatic rhabdomyolysis with short-term '
'alcohol intoxication—a case report. Clin. Case Rep. 3, 769–772. '
'https://doi.org/10.1002/ccr3.326 (2015).',
'journal-title': 'Clin. Case Rep.'},
{ 'key': '74084_CR32',
'doi-asserted-by': 'publisher',
'first-page': '753',
'DOI': '10.1007/BF03012146',
'volume': '45',
'author': 'T Nishiyama',
'year': '1998',
'unstructured': 'Nishiyama, T., Yokoyama, T. & Hanaoka, K. Liver function after '
'sevoflurane or isoflurane anaesthesia in neurosurgical patients. Can. J. '
'Anaesth. 45, 753–756. https://doi.org/10.1007/BF03012146 (1998).',
'journal-title': 'Can. J. Anaesth.'},
{ 'key': '74084_CR33',
'first-page': '25',
'volume': '43',
'author': 'AG Gil',
'year': '2004',
'unstructured': 'Gil, A. G., Silvan, G., Illera, M. & Illera, J. C. The effects of '
'anesthesia on the clinical chemistry of New Zealand White rabbits. '
'Contemp. Top. Lab. Anim. Sci. 43, 25–29 (2004).',
'journal-title': 'Contemp. Top. Lab. Anim. Sci.'},
{ 'key': '74084_CR34',
'doi-asserted-by': 'publisher',
'first-page': '238',
'DOI': '10.1111/acer.13291',
'volume': '41',
'author': 'RR MacLean',
'year': '2017',
'unstructured': 'MacLean, R. R., Valentine, G. W., Jatlow, P. I. & Sofuoglu, M. '
'Inhalation of alcohol vapor: measurement and implications. Alcohol Clin. '
'Exp. Res. 41, 238–250. https://doi.org/10.1111/acer.13291 (2017).',
'journal-title': 'Alcohol Clin. Exp. Res.'},
{ 'key': '74084_CR35',
'doi-asserted-by': 'publisher',
'first-page': '388',
'DOI': '10.1016/j.pt.2017.12.007',
'volume': '34',
'author': 'C Schwartz',
'year': '2018',
'unstructured': 'Schwartz, C., Hams, E. & Fallon, P. G. Helminth modulation of lung '
'inflammation. Trends Parasitol. 34, 388–403. '
'https://doi.org/10.1016/j.pt.2017.12.007 (2018).',
'journal-title': 'Trends Parasitol.'},
{ 'key': '74084_CR36',
'doi-asserted-by': 'publisher',
'first-page': '176',
'DOI': '10.1016/j.pt.2010.11.008',
'volume': '27',
'author': 'IA Sutherland',
'year': '2011',
'unstructured': 'Sutherland, I. A. & Leathwick, D. M. Anthelmintic resistance in nematode '
'parasites of cattle: a global issue?. Trends Parasitol 27, 176–181. '
'https://doi.org/10.1016/j.pt.2010.11.008 (2011).',
'journal-title': 'Trends Parasitol'},
{ 'key': '74084_CR37',
'doi-asserted-by': 'publisher',
'first-page': '1670',
'DOI': '10.1056/NEJMc1210168#SA2',
'volume': '367',
'author': 'CJ Chaccour',
'year': '2012',
'unstructured': 'Chaccour, C. J. & Del Pozo, J. L. Case 23–2012: a man with abdominal '
'pain and weight loss. N. Engl. J. Med. 367, 1670–1671. '
'https://doi.org/10.1056/NEJMc1210168#SA2 (2012).',
'journal-title': 'N. Engl. J. Med.'},
{ 'key': '74084_CR38',
'doi-asserted-by': 'publisher',
'first-page': '1292',
'DOI': '10.1007/s00134-005-2725-y',
'volume': '31',
'author': 'JI Salluh',
'year': '2005',
'unstructured': 'Salluh, J. I. et al. Successful use of parenteral ivermectin in an '
'immunosuppressed patient with disseminated strongyloidiasis and septic '
'shock. Intensive Care Med. 31, 1292 (2005).',
'journal-title': 'Intensive Care Med.'},
{ 'key': '74084_CR39',
'doi-asserted-by': 'publisher',
'first-page': '122',
'DOI': '10.4269/ajtmh.2005.73.122',
'volume': '73',
'author': 'J Pacanowski',
'year': '2005',
'unstructured': 'Pacanowski, J. et al. Subcutaneous ivermectin as a safe salvage therapy '
'in Strongyloides stercoralis hyperinfection syndrome: a case report. Am. '
'J. Trop. Med. Hyg. 73, 122–124 (2005).',
'journal-title': 'Am. J. Trop. Med. Hyg.'},
{ 'key': '74084_CR40',
'doi-asserted-by': 'publisher',
'first-page': '700',
'DOI': '10.1111/j.1472-8206.2010.00896.x',
'volume': '25',
'author': 'X Zhang',
'year': '2011',
'unstructured': 'Zhang, X. et al. Protective effect of abamectin on acute lung injury '
'induced by lipopolysaccharide in mice. Fundam. Clin. Pharmacol. 25, '
'700–707. https://doi.org/10.1111/j.1472-8206.2010.00896.x (2011).',
'journal-title': 'Fundam. Clin. Pharmacol.'},
{ 'key': '74084_CR41',
'doi-asserted-by': 'publisher',
'first-page': '1263',
'DOI': '10.15252/emmm.201404084',
'volume': '6',
'author': 'A Melotti',
'year': '2014',
'unstructured': 'Melotti, A. et al. The river blindness drug Ivermectin and related '
'macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. '
'EMBO Mol. Med. 6, 1263–1278. https://doi.org/10.15252/emmm.201404084 '
'(2014).',
'journal-title': 'EMBO Mol. Med.'}],
'container-title': 'Scientific Reports',
'original-title': [],
'language': 'en',
'link': [ { 'URL': 'https://www.nature.com/articles/s41598-020-74084-y.pdf',
'content-type': 'application/pdf',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://www.nature.com/articles/s41598-020-74084-y',
'content-type': 'text/html',
'content-version': 'vor',
'intended-application': 'text-mining'},
{ 'URL': 'https://www.nature.com/articles/s41598-020-74084-y.pdf',
'content-type': 'application/pdf',
'content-version': 'vor',
'intended-application': 'similarity-checking'}],
'deposited': { 'date-parts': [[2022, 12, 6]],
'date-time': '2022-12-06T21:17:19Z',
'timestamp': 1670361439000},
'score': 1,
'resource': {'primary': {'URL': 'https://www.nature.com/articles/s41598-020-74084-y'}},
'subtitle': [],
'short-title': [],
'issued': {'date-parts': [[2020, 10, 13]]},
'references-count': 41,
'journal-issue': {'issue': '1', 'published-online': {'date-parts': [[2020, 12]]}},
'alternative-id': ['74084'],
'URL': 'http://dx.doi.org/10.1038/s41598-020-74084-y',
'relation': { 'has-preprint': [ { 'id-type': 'doi',
'id': '10.21203/rs.3.rs-64501/v2',
'asserted-by': 'object'},
{ 'id-type': 'doi',
'id': '10.21203/rs.3.rs-64501/v1',
'asserted-by': 'object'}]},
'ISSN': ['2045-2322'],
'subject': [],
'container-title-short': 'Sci Rep',
'published': {'date-parts': [[2020, 10, 13]]},
'assertion': [ { 'value': '13 July 2020',
'order': 1,
'name': 'received',
'label': 'Received',
'group': {'name': 'ArticleHistory', 'label': 'Article History'}},
{ 'value': '25 September 2020',
'order': 2,
'name': 'accepted',
'label': 'Accepted',
'group': {'name': 'ArticleHistory', 'label': 'Article History'}},
{ 'value': '13 October 2020',
'order': 3,
'name': 'first_online',
'label': 'First Online',
'group': {'name': 'ArticleHistory', 'label': 'Article History'}},
{ 'value': 'The authors declare no competing interests.',
'order': 1,
'name': 'Ethics',
'group': {'name': 'EthicsHeading', 'label': 'Competing interests'}}],
'article-number': '17073'}